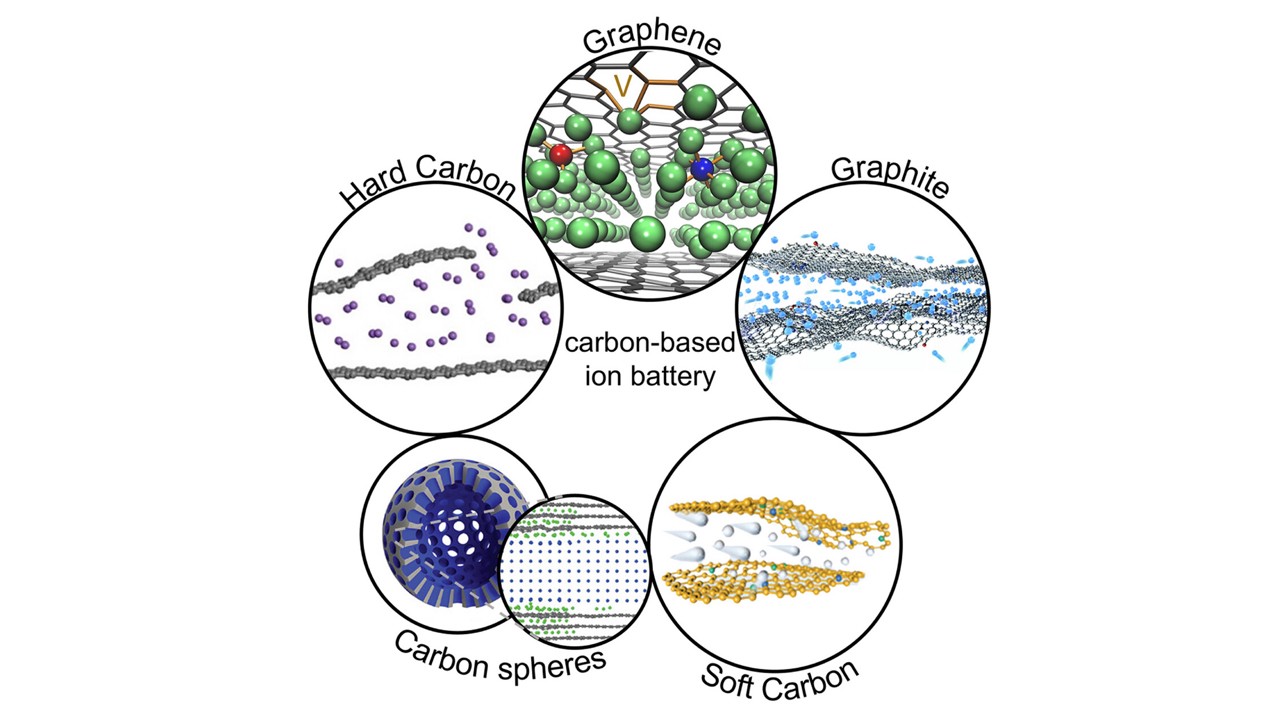
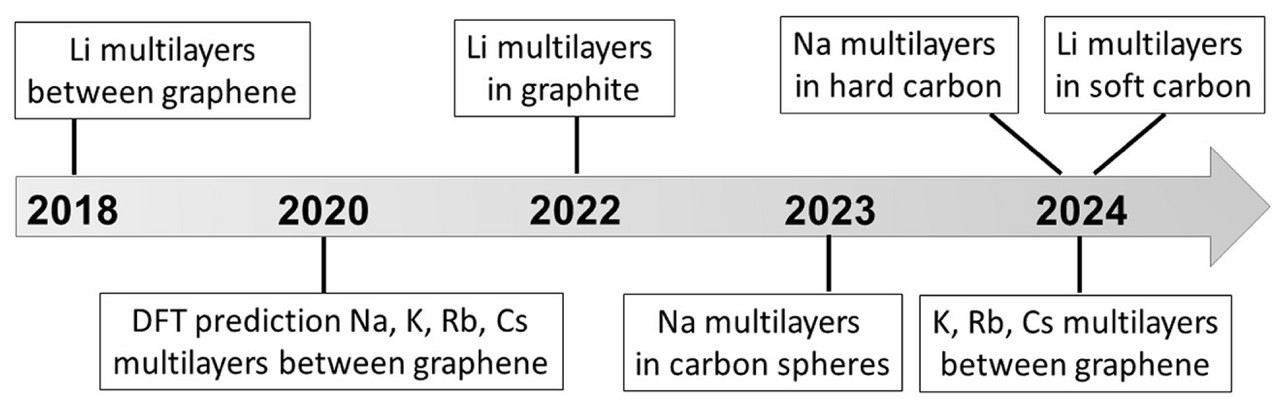
This new paradigm offers several advantages. First, packing multiple metal layers instead of just one into the anode dramatically increases the storage capacity. According to theoretical calculations, a graphene anode with four lithium layers could have up to three times the capacity of the most advanced modern graphite anode.
Second, the nanochannels and pores that are specifically designed for the carbon matrix act as a “superhighway” for ions. This concept was demonstrated with hard carbon-based sodium-ion batteries, which retained 83% of their capacity after 3,000 ultrafast charging cycles.
Safety is equally important. The growth of needlelike metal dendrites is the primary cause of battery fires. This new technology solves the problem by causing the metal to deposit and dissolve within the stable carbon frame rather than dangerously on its surface, eliminating the risk of short circuits.
“Our study breaks new ground in energy materials science,” the lead author of the paper, Senior Research Scientist Ilya Chepkasov from Skoltech’s Energy Transition Center, commented. “We have systematized the facts proving that nature allows us to ‘pack’ ions into carbon much more densely than was previously believed possible. The key lies in designing the carbon carrier by creating atomic channels in graphite, controlling the size of nanopores in hard carbon, or introducing open mesochannels into carbon spheres. Each of these architectures directs ions along an optimal path, enabling the formation of stable multilayered structures that account for this exceptional performance.”
The team’s findings outline a clear path from laboratory discovery to industrial production. Methods such as molecular tunneling with ammonia to modify graphite and complex hard carbon synthesis from biomass can already be used today to create functional anode prototypes. However, widespread adoption will depend on meeting a set of interconnected challenges:
Developing advanced simulation methods, including AI-based tools, to accurately predict material properties.
Improving experimental techniques to enable real-time observation of atomic-level processes inside a working battery.
Optimizing the synthesis processes by scaling them up and reducing their cost.
“We already understand the physical principles behind this discovery, and scientists working in the field have the first working specimens ready. In the next phase, we need to transition from unique lab samples to cost-effective, reliable industrial production. Combining computational science, advanced analytics, artificial intelligence, and chemical synthesis provides a toolkit for translating this breakthrough research into commercial products that will shape the future of energy,” said Alexander Kvashnin, a co-author of the study and a professor at the Energy Transition Center of Skoltech.
Research on incorporating multilayered alkali metals into carbon anodes is essential to overcoming the key limitations of modern battery technology. These studies could result in a new generation of energy storage devices offering high capacity, superfast charging, enhanced safety, and lower cost.